All News
Team connects genetic variant to poor outcomes after certain cancer treatment
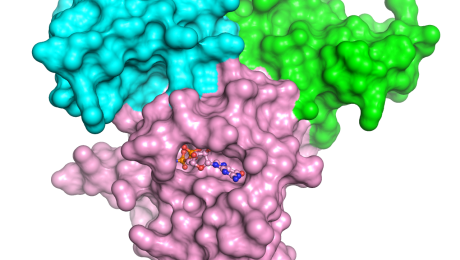
Basic Science ProgramPosted 2/17/2022Scientists have identified a genetic variant that can predict whether immunotherapy drugs called immune checkpoint inhibitors, used to treat cancer, might fail in certain patients.
The team’s findings, which appear in Lancet Oncology, point to HLA-A*03, an allele (a form of a gene) found on chromosome 6 of human DNA. The presence of HLA-A*03 in cancer patients’ DNA was…
Three Frederick National Laboratory teams awarded 2021 NIH director’s awards
Protein Expression Laboratory, Clinical Monitoring Research Program, Terri BrayPosted 2/14/2022The National Institutes of Health (NIH) awarded three Frederick National Laboratory teams with NIH Director’s Awards for innovative approaches to COVID-19-related research that advances knowledge and enhances health.
“It is a tremendous honor for three Frederick National Laboratory teams to be nominated by three distinct entities within NIH. I am grateful to the NIH for…
First Women in Science Speak event celebrates International Women and Girls in Science Day
Partnership Development OfficePosted 2/11/2022Feb. 11 was International Women and Girls in Science Day. To celebrate, we co-hosted a panel discussion with Woman to Woman Mentoring where successful women in science discussed their chosen career paths, allyship and mentorship in science and strategies for advancement.
Keynote speaker
Carla Williams, Ph.D.
Associate Professor of Medicine &…
Nanotechnology enables potential novel imaging agent for early pancreatic cancer detection
Nanotechnology Characterization Laboratory, Laboratory Animal Sciences ProgramPosted 2/10/2022Frederick National Laboratory (FNL) scientists, led by Stephan Stern, Ph.D., and their colleagues have created a novel imaging agent that, with further development, might detect deadly pancreatic cancer at its earliest, most-treatable stages and thereby improve the prognosis for patients.
Pancreatic cancer has a high mortality rate because it is usually diagnosed late,…
Machine learning-backed model reveals new insights about RAS biology not possible with traditional experiments
Posted 1/26/2022A key to understanding KRAS-driven cancers and the role of mutant RAS proteins and to discover new therapeutic possibilities is to better understand how RAS behaves on the cell membrane.
Investigating RAS in the context of membranes is somewhat challenging using conventional computational or experimental techniques. In a partnership with researchers from three Department…
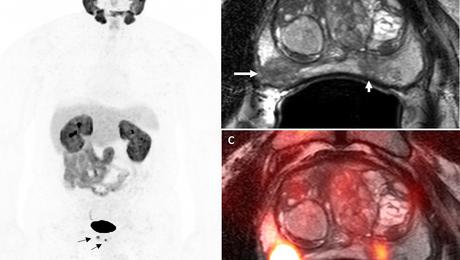
PET imaging might improve prostate cancer diagnosis and treatment
Clinical Research DirectoratePosted 1/24/2022A pilot study suggests a near real-time method of imaging prostate cancer to aid in organ-sparing treatments, and an innovative as well as accurate way to see whether the cancer has spread.
These dual findings stem from research reported in Molecular Imaging and Biology on a relatively new radiotracer used in dynamic positron emission tomography (PET) to detect levels…
Public-private network ensures accuracy of COVID-19 antibody tests available in US market
Vaccine, Immunity, and Cancer DirectoratePosted 1/20/2022 Frederick National Laboratory for Cancer Research (FNL) plays a central role in a public-private network that has kept scores of substandard COVID-19 blood antibody tests out of circulation and helped ensure that marketed tests give accurate results.
This critical pandemic response is detailed in a scientific study published January 12 in the journal Microbiology…
Growing cancer cells in 3D -- not just 2D -- might improve preclinical drug development
Antibody Characterization Laboratory, Cancer Research Technology ProgramPosted 1/18/2022One way to improve the success rate for new cancer drugs might be to test them in a three dimensional (3D) environment that mimics real life better than the two-dimensional (2D) set-up of a flat culture dish, according to a new study that examined thousands of lung cancer proteins grown under both conditions.
About 95 percent of experimental drugs for lung cancer fail…
Scientists develop new concept for hard-to-treat cancers like mesothelioma
Laboratory Animal Sciences ProgramPosted 1/13/2022Think of a tumor and you might envision a disorganized mass. But tumors can also form a thin sheet over a surface. These can be hard to treat, especially when the surface has wrinkles and crevices. But there may be a new approach.
Solid tumors can be successfully removed with surgery and residual cells destroyed. But cancers like the asbestos-related lung malignancy…
Partnership with FCC aims to support the next generation of STEM in Frederick
Partnership Development OfficePosted 1/11/2022
The Frederick National Laboratory for Cancer Research (FNL) signed a new memorandum of understanding (MOU) with Frederick Community College (FCC) to establish a closer partnership to support the future science, technology, engineering and mathematics (STEM) workforce in Frederick.
“As a scientific organization, we are eager to connect with and inspire the next…