When nanoparticle-based mRNA COVID-19 vaccines were made available in December 2020 and the media began to report the incidence of rare but troubling anaphylaxis, nanomedicine experts including Frederick National Laboratory’s Marina Dobrovolskaia recognized those hypersensitivity reactions.
“Everything they reported symptomatically sounded so familiar to us from our work with nanomedicines,” said Dobrovolskaia, co-director and the head of the immunology section of the National Cancer Institute’s Nanotechnology Characterization Laboratory at FNL.
The nanomedicine field has long studied why these reactions occur after the administration of certain type of nanoformulations (for example, PEGylated liposomal doxorubicin) and how to avoid them. Dobrovolskaia and colleagues wanted to share what they’ve learned with their fellow scientists.
In a review article published in Nature Nanotechnology, the team discussed factors that contribute to immunological responses to nanomedicines, including known and potential molecular and cellular mechanisms underlying some patients’ hypersensitivity.
“The team felt we had a lot of knowledge over the past 15 years and wanted to come out and speak about our experience with nanomedicines to inform the vaccine field,” said Dobrovolskaia, corresponding author on the paper.
mRNA vaccine sensitivity mirrors nanomedicine reactions
Some physical reaction following a vaccine is not uncommon, Dobrovolskaia said, and it demonstrates the person’s immune system is doing its job.
“What we want to understand is how to make these expected immune responses at a level that people don’t notice them,” she said.
The Pfizer-BioNTech and Moderna COVID-19 formulations are the first vaccines to use the mRNA and nanoparticle technologies. The authors’ close examination of the adverse events following administration of COVID mRNA vaccines revealed that many resemble infusion reactions commonly experienced in response to intravenously administered established nanomedicines.
Reactions include rash, chills, chest pain, elevated heart rate, high or low blood pressure, and anaphylaxis, a sudden, severe and life-threatening reaction marked by the closing of the airway, leading to difficulty breathing.
But unlike infusion reactions to nanomedicines, anxiety was suggested among the causes of hypersensitivity reactions in vaccine recipients. Understanding the mechanisms that underlie the hypersensitivity reactions would help the medical community develop means to manage these reactions and prevent major side effects and deaths, the authors said, and so reduce people’s anxiety and fears over receiving the vaccine.
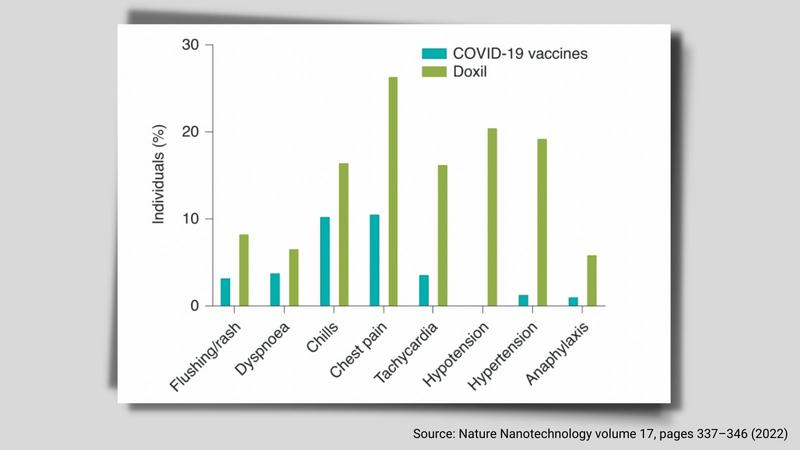
The percent of individuals on this graph refers to the proportion of individuals with this adverse effect among 100 reports entered in VAERS for the Pfizer-BioNTech and Moderna SARS-CoV-2 vaccines by 8 January 2021, or registered for Doxil by the FDA adverse event reporting system between 1995 (FDA approval of this formulation) and 2021.
Immune responses required for vaccine efficacy
The potential mechanisms involve the activation of both systemic and intracellular complement; mast cells and basophils; cytokine production by the cells present at the injection site and involved in the immune response; platelets, bradykinin and coagulation systems; oxidative stress; variability in the human leukocyte antigen; and some genetic conditions such as common variable immunodeficiency.
These mechanisms are triggered by at least one or all components of the vaccine (LNP carrier, mRNA, antigen expressed from the mRNA, and excipients). These responses are required for vaccine efficacy, but also contribute to immune-mediated side effects in sensitive individuals.
Sharing knowledge to improve vaccines
The authors said they recognize the need for high-risk people to be immunized and boosted, and for reliable tools to predict adverse reactions to mRNA vaccines.
“Our intent was to synthesize the knowledge that the vaccine field could use to improve the quality of the vaccines,” Dobrovolskaia said. “Why should the vaccine field start from scratch to find out what is happening when there is a parallel field that can share the knowledge?”
As a national resource, the Nanotechnology Characterization Laboratory is charged with providing its expertise to benefit the scientific community.
“We are doing exactly what we are expected to do,” Dobrovolskaia said. “We are seeing the problem and educating people. The overall goal is to improve the quality of the vaccines.”
Dobrovolskaia’s co-authors on the paper include world-renowned experts Janos Szebeni, Gert Storm, Julia Y. Ljubimova, Mariana Castells, Elizabeth J. Phillips, Keren Turjeman, Yechezkel Barenholz, and Daan J.A. Crommelin from internationally recognized and respected academic institutions (Terasaki Research Institute, Harvard and Vanderbilt Universities in the US, Semmelweis University in Hungary, Hebrew University in Israel, Utrecht University and University of Twente in Netherlands, and National University of Singapore.
Media Inquiries
Mary Ellen Hackett
Manager, Communications Office
301-401-8670
