A grim statistic is driving Steven Rosenberg’s mission to find new cancer treatments—and his partnership with Frederick National Laboratory (FNL) to do so.
“Every year in the United States, about 600,000 people die of cancer, 90% of whom die of the solid epithelial cancers,” said Rosenberg, the M.D., Ph.D., chief of the National Cancer Institute’s (NCI) Surgery Branch, head of the Tumor Immunology Section at NCI’s Center for Cancer Research.
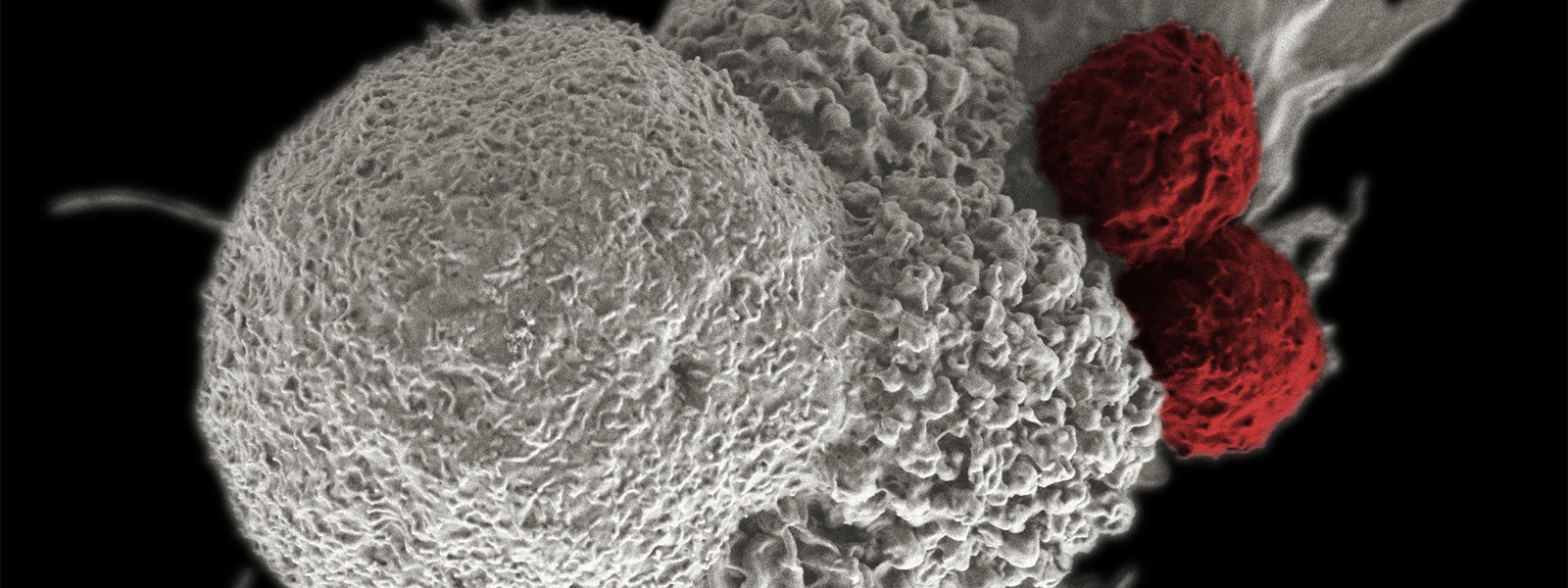
The Surgery Branch conducts clinical trials focused on treating patients with advanced metastatic cancers. Its staff is testing immunotherapies in which a patient’s own white blood cells are removed and genetically modified to embed a molecule called a “T-cell receptor” (TCR) on their surfaces that identifies that patient’s particular cancer mutation that drives growth of that cancer. These cells are multiplied outside the body to become billions, creating a sort of custom TCR cancer-fighting “army” built from the patient’s own cells that gets infused back into the patient.
“Everything that we’re doing is attempting to develop better cancer treatments. We’re working around the clock to try to develop more effective treatments that differ from surgery, radiation, and chemotherapy, that take advantage of the body’s own natural immune defense mechanisms to fight the cancer,” Rosenberg added.
Immunotherapy is the newest frontier of cancer treatments, and Rosenberg, the Surgery Branch, and their FNL collaborators are among those at the forefront. The Surgery Branch is a laboratory and clinical research unit that develops cancer immunotherapies.
Employees from FNL’s Clinical Research Directorate are embedded within the Surgery Branch. They operate most of the Vector Production Facility, the whole Quality Assurance unit, some of the Cell Production Facility, and other groups. Their support has spanned several areas in recent years and substantially advanced the branch’s efforts.
“It’s an exciting time to be supporting immunotherapy research at its highest level within the Surgery Branch,” said Adrian Cuenca, Ph.D., a process development scientist in the Clinical Research Directorate.
Collaborating on immunotherapy products
Two arms of the Surgery Branch with FNL employees, the Vector Production Facility and the Cell Production Facility, are responsible for producing the therapeutic products that will be tested in clinical trials.
“[FNL] is uniquely positioned to staff and manage [cell production and vector production] because of our long history of operating two much larger manufacturing facilities in Frederick—the Vaccine Clinical Materials Program and the Biopharmaceutical Development Program—and … long history of providing cGMP [current Good Manufacturing Practice] quality cellular therapy manufacturing of products given to patients in a safe manner and recognized as such by the FDA [Food and Drug Administration],” said Barry Gause, M.D., FNL’s chief medical officer and head of the Clinical Research Directorate.

The Vector Production Facility, headed by an NCI employee and staffed by FNL employees overseen by Cuenca, produces retroviral vectors, a tool used by the Cell Production Facility to modify patients’ cells to have the right TCRs for their treatment.
“These viruses are vehicles where we introduce particular genes that will make a particular protein. In this case, it’s a T-cell receptor. These T-cell receptors are cloned … these viruses grow up and we harvest them, and then we hand [this viral vector product] off to our sister facility, the Cell Production Facility,” said Cuenca.
Meanwhile, the Cell Production Facility takes patient cells harvested by the branch’s Apheresis Unit and the vectors produced by the Vector Production Facility to manipulate the patient cells into a customized treatment.
“The retrovirus inserts this TCR into [these patient cells] … and now you have an army [of T cells] … expressing the same TCR … [and] that specifically recognizes a mutation in the patient’s own cancer cells,” Cuenca explained.
The two units work together seamlessly to produce the final infusion given to the patients in the clinical trials.
“Everybody wants to support the work that’s going on, and it’s truly, truly collaborative,” said Jack Fisher, a Surgery Branch quality assurance manager in FNL’s Clinical Research Directorate.
Digging into the details
Alongside production, safety is another essential part of making the therapies.
“The need to have intensive quality assurance that we administer to patients is critical,” said Rosenberg.
FNL employees were first brought into the Surgery Branch to create the Quality Assurance unit. They handle all aspects of its operation, making sure measures are in place to protect patients and product safety and performance according to cGMP and FDA regulations.
“The bottom line is that the Quality Assurance team is charged with providing an independent oversight. ... Making sure that we’re safe and being compliant and we’re fulfilling the requirements for regulations, which are really designed to ensure safety for the public. And in this case, for the patients in our clinical trials,” said Fisher.
“Everything that goes into the product, that touches the product, has to be reviewed and maintained at a certain level,” said Michelle Chaikin, a Surgery Branch quality assurance manager in FNL’s Clinical Research Directorate.
That includes monitoring special requirements for production and testing, for the employees handling the products, and even for the building housing the production and treatment.
The work is meticulous.
“You have to be really detail-oriented, but we’re lucky we have a really great team. We work really well together, so it’s fun,” Chaikin said.
Before FNL employees were hired to build the unit, there had been no independent quality assurance group for the Surgery Branch. The unit’s independence helps establish standard procedures that support regulatory compliance, enforce accountability, and avoid conflicts of interest.
It also doesn’t hurt cooperation. Fisher says the Surgery Branch works so collaboratively that people barely notice the divide between government and contractor employees.
“We all work so seamlessly together that I sort of forget who’s who. … It’s definitely a whole group collaborative effort,” said Chaikin.
Growing the team and production capabilities
The Quality Assurance team paved the way for the rest of the FNL employees in the Surgery Branch, and FNL’s involvement has only grown. There are multiple job openings for FNL employees to work in the Surgery Branch, with more likely to come.
One reason for the growth is the addition of a new building called “T30,” a fully cGMP-certified facility for production and patient infusions. Planning for the building began in 2017, and ground was broken in 2019. The Quality Assurance team has been involved from the start, ensuring throughout the regulatory testing that the building will be compliant.
“[FNL] is very proud to play a role in supporting the development of [Dr. Rosenberg’s] new and exciting projects, which will continue to have a major impact on cancer care,” said Gause.
The building was designed because of the previous facility’s “gradual degeneration to beyond what procedural controls could mitigate,” said Chaikin. “This new facility will address all those things. … This facility is much larger and has much more robust controls in place to prevent any kind of contamination.”
Additionally, “It will eventually allow us to more than double our throughput,” Fisher said.
This may at some point require bolstering the team even more. For now, they’re busy accommodating final corrective and preventive actions to address some minor concerns and questions that came up during the building’s inspection. Pending a positive review of those changes, the building will, hopefully, open to manufacturing this calendar year.
“It’s just a world of difference from what we were to what we are now. … It’s really exciting for us to be finishing up this process,” said Fisher.
The most exciting part is how much the new building will impact the clinical research, and ultimately, patient care.
“FNL plays a vital role in our activities and has been very helpful to us over the years. … The help that we get from FNL is an important part of that whole effort,” said Rosenberg.
Media Inquiries
Mary Ellen Hackett
Manager, Communications Office
301-401-8670
