
The Frederick National Laboratory (FNL) has begun building out new production space that will triple its capacity to manufacture cell and gene therapies to support cancer clinical trials.

BDP manufacturing staff use a Miltenyi Prodigy for CAR T-cell production. Incubators seen in the background are available for non-Prodigy based cell therapy production.
The manufacture of cell and gene therapies, such as chimeric antigen receptor (CAR) T cell therapies, is expensive, labor intensive, complex, and highly regulated. Each therapy is custom-made to address the needs of an individual patient, so mass production is not possible.
“It’s not a product in the classic sense,” said George Mitra, director of the Biopharmaceutical Development Program (BDP) at FNL. “It’s more like clinical research.”
This makes it difficult to conduct large, multicenter clinical trials or to compare a therapy developed at one center with those made at other sites. “So, it’s important to develop collaborations around centralized manufacturing,” he said.
To make a CAR T cell treatment, blood cells from a patient are harvested using a technique called apheresis. From these cells, a population of T cells is isolated. The T cells, which are critical to fighting foreign invaders, are genetically engineered to recognize and attack the patient’s own cancer. The engineered cells are replicated in the manufacturing suite until millions of them are ready for injection back into the patient.
To meet a growing demand for cell and gene therapies directed against cancer, the National Cancer Institute (NCI) tasked FNL with setting up a production facility at the Advanced Technology Research Facility. The BDP, sponsored by the NCI Division of Cancer Treatment and Diagnosis, developed the resources and capability to turn an existing virus production suite into a CAR T cell manufacturing facility in early 2020 and began supplying the immunotherapies to multiple clinical locations for early-phase trials.
A cell therapy suite at the Advanced Technology and Research Facility in Frederick with multiple Biosafety hood available for different viral vectors when necessary.

“NCI anticipates an increased need for GMP [Good Manufacturing Practice] space for cell therapy and related products, so they commissioned us for much larger facility,” said Doug Gaum, BDP’s director of Quality Assurance.
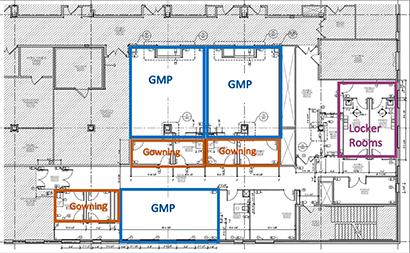
NCI will fund the renovation of existing laboratory and office space into three new manufacturing suites for a total of 4,155 square feet (2,015 square feet of clean-room suites and 2,140 square feet of support space). Construction on the third floor of the ATRF’s B wing is getting under way with a target completion date in November 2021, Gaum said.
So far, two therapies are lined up for production at the existing CAR T cell suite, Mitra said. Therapies have already been manufactured for three patients with advanced acute myeloid leukemia. Another type of CAR T cell treatment has recently been approved for phase I clinical trials and manufacturing to support that trial will begin soon for patients with pediatric sarcoma and neuroblastoma.
As a related, longer-term research project, FNL will explore using the gene editing technique CRISPR (clustered regularly interspaced short palindromic repeats) to engineer novel cell therapy products directed at fighting cancer, Mitra said.
Renovations underway at the Advanced Technology and Research Facility in Frederick. cGMP Manufacturing capacity will be increased with the addition of 3 suites that can be utilized for cell therapy or production for transduction vectors (lentivirus, gamma retrovirus, AAV).
Manufacturing Logistics for Autologous Cell Therapy Products. The Biopharmaceutical Development Program can now deliver customized cancer-fighting CAR-T cell therapy to pediatric patients in approximately 21 days.
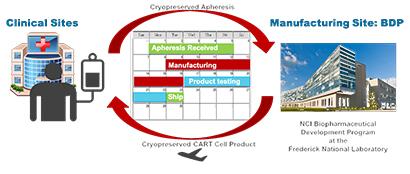
Manufacturing Logistics for Autologous Cell Therapy Products. The Biopharmaceutical Development Program can now deliver customized cancer-fighting CAR-T cell therapy to pediatric patients in approximately 21 days.
Media Inquiries
Mary Ellen Hackett
Manager, Communications Office
301-401-8670
