FREDERICK, Md. -- CRISPR is one of the most widely used technologies in the nascent quest to edit the human genome, and the precision “instrument” that makes it so effective is Cas9, a programmable enzyme harnessed from bacterial cells that cuts DNA strands at specific targets and replaces one gene with another.
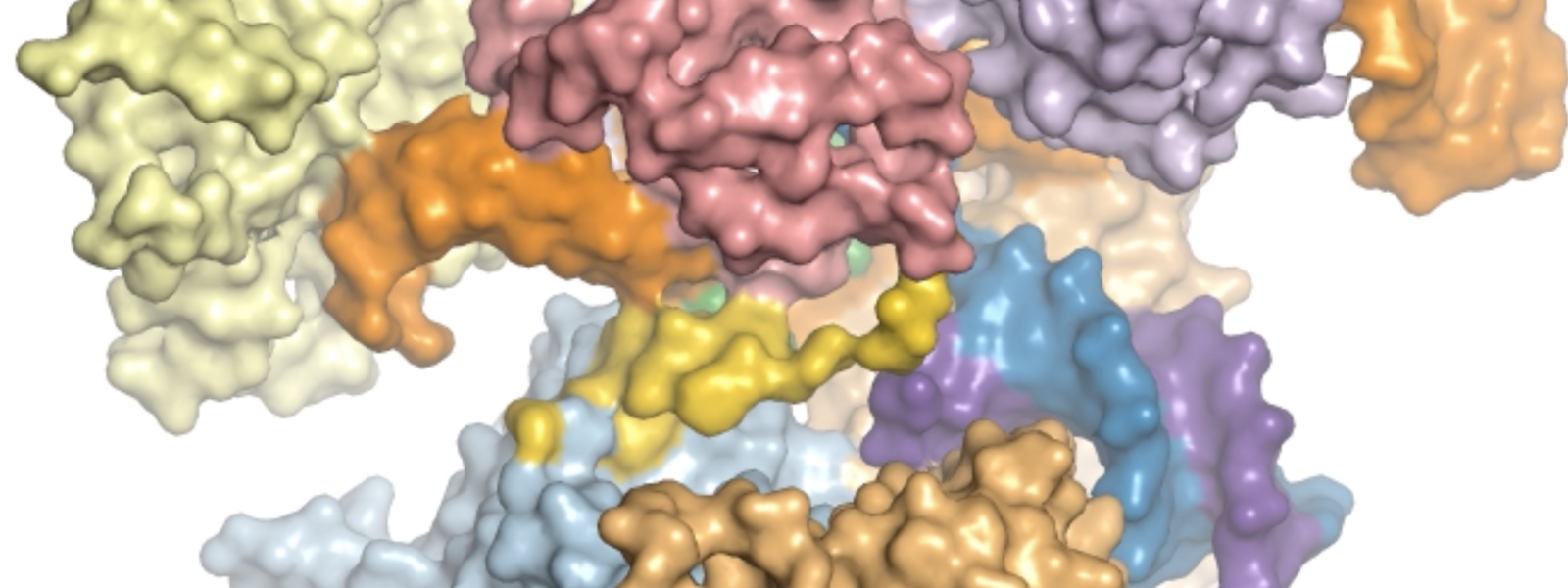
In a July 8 paper published in Nature Structural and Molecular Biology, scientists who used a near-atomic resolution cryo-electron microscope to visualize each part of the editing process reveal just how Cas9 makes that happen. It’s the first time the structure of Cas9 has been viewed in action.
“Now that we know exactly how that cut takes place, we can engineer it,” said co-author Sriram Subramaniam, Ph.D., the founding director of the National Cryo-Electron Microscopy Facility at the Frederick National Laboratory for Cancer Research. Subramaniam now serves as its scientific advisor and is a professor at the University of British Columbia.
Engineering Cas9 might involve speeding or slowing the cutting process or otherwise making it more efficient. As bioethicists and others worldwide contend with the ethical and legal complexities surrounding of genetic engineering, Subramaniam said the discoveries detailed in his paper could enable advances to improve the efficacy and safety CRISPR. “Now that we understand it better, we have a chance to improve it,” he said.
Cryo-EM technology involves imaging radiation-sensitive specimens in a transmission electron microscope under cryogenic conditions. Rapidly freezing the specimens to a frozen-hydrated state preserves them, allowing for optimal viewing with the high-powered electron microscope.
This animation created with cryo-EM structures depicts the pathway of the CRISPR Cas9 enzyme as it cuts DNA for gene editing. Animation courtesy of Xing Zhu, Miljan Simonovic and Sriram Subramaniam.
In an editorial accompanying the paper, David Taylor, Ph.D., of the University of Texas at Austin said the structures the authors captured via cryo-EM “provide an unprecedented series of SpCas9 ‘snapshots’” as it moves through the process of editing DNA. SpCas9, or Streptococcus pyogenes Cas9, is another term for Cas9 that includes the strain of bacteria from which it originates.
Subramaniam and his colleagues initiated the study while he was at the National Cancer Institute’s Center for Cancer Research, and completed it after moving to the University of British Columbia. Co-author Alan Merk, Ph.D., worked in Subramaniam’s CCR lab and is now with the cryo-EM facility at FNL where he’s part of a group seeking to further develop cryo-EM technology.
By editing DNA at precise locations, CRISPR-Cas9 technology can permanently modify targeted genes. This process may make it possible to treat and prevent a range of genetic causes of human disease.
By Mary Ellen Hackett, staff writer
Media Inquiries
Mary Ellen Hackett
Manager, Communications Office
301-401-8670
