The Frederick National Laboratory’s Nanotechnology Characterization Laboratory recently supported a first-of-its-kind study examining whether nanoparticles used for drug delivery, vaccines, and biomedical imaging were toxic over an extended period of time.
The study, led by the Utah Center for Nanomedicine at The University of Utah, looked at the toxicity of certain nanoparticles known as silica nanoparticles, ultrafine substances thousands of times smaller than a human hair, over the course of a year. Most prior research into their potential toxicity focused on short-term toxicity.
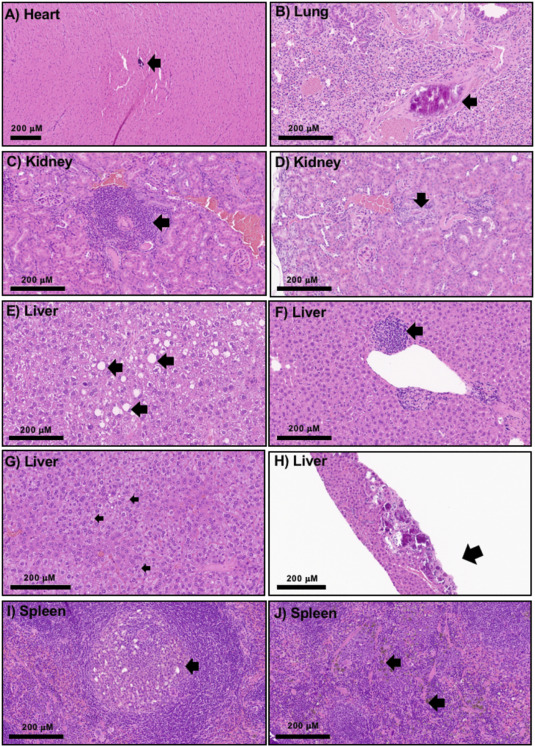
While treatments using nanoparticles may decrease disease symptoms without appreciable toxicity in the weeks that follow, that does not mean they are safe over a longer time frame. Indeed, as the study’s authors note, adverse effects from chronic, or long-term, exposure are one of the primary reasons that pharmaceuticals get recalled.
“At the end of the day, the drug or imaging agent carrier should have minimal or no toxic effects and be eliminated from the body in a timely fashion,” said Raziye Mohammadpour, Ph.D., and Hamidreza Ghandehari, Ph.D., the paper’s first and corresponding authors, respectively. “But the majority of studies in this regard have investigated the effects of these particles in terms of days and weeks, or just on cells in culture media. We conducted these studies over one year in mice and with a systematic variation in size and porosity.”
The team found that, after one year, exposure to several different silica nanoparticles was tolerated without statistically significant changes in morbidity, body weight changes, or hematological profiles, including cell blood count and blood biomarker indices, compared to a control group given a saline solution. The findings will prove valuable for scientists and clinicians establishing guidelines for the safe and effective use of silica nanoparticles in the clinic.
Seeking additional expertise, the study’s authors reached out to Marina Dobrovolskaia, Ph.D., an immunologist in the Nanotechnology Characterization Laboratory, for advice about the study’s design and protocols as well as interpretation of their results. NCL is a shared resource dedicated to nanoparticle characterization, education, and knowledge sharing related to nanomaterials and their use in cancer research.
“Worldwide, there are very few scientists who have expertise at the interface of nanoparticle characteristics and immunotoxicity. Dr. Dobrovolskaia is very knowledgeable in this area and has brought a very unique perspective to the field,” said Mohammadpour and Ghandehari.
Dobrovolskaia helped train the University of Utah team on assays to evaluate hemolysis (the rupturing of red blood cells) and complement activation (an overactive immune state that drives inflammation). She also provided advice for the interpretation of the blood and immunotoxicity results and helped write the manuscript.
“Our main mission is to support the characterization of nanoparticles and advance the science to help translate these materials into the clinic,” Dobrovolskaia said, “and investigators like Dr. Ghandehari are helping the research community tremendously by applying their unique expertise to synthesize these materials, perform the structure-activity relationship studies, and analyze toxicity in a systematic way so that we, as a community, can learn from it.”
This is not the first time that Dobrovolskaia has worked with Ghandehari. In fact, their professional relationship spans many years. In the past, she has helped conduct a workshop on the immunotoxicity of nanoparticles at The University of Utah, participated in a thesis committee for one of his graduate students, and co-authored several manuscripts with his group. But this latest collaboration stands out for one particular reason.
“In this case,” added Dobrovolskaia, “I have been strongly motivated by one of the national lab’s missions to not only provide experimental resources like the core services, but also to assist the extramural researchers with advancing their science. It’s a win-win situation that benefits all nanomedicine stakeholders—researchers, clinicians, and patients alike.”
Media Inquiries
Mary Ellen Hackett
Manager, Communications Office
301-401-8670
