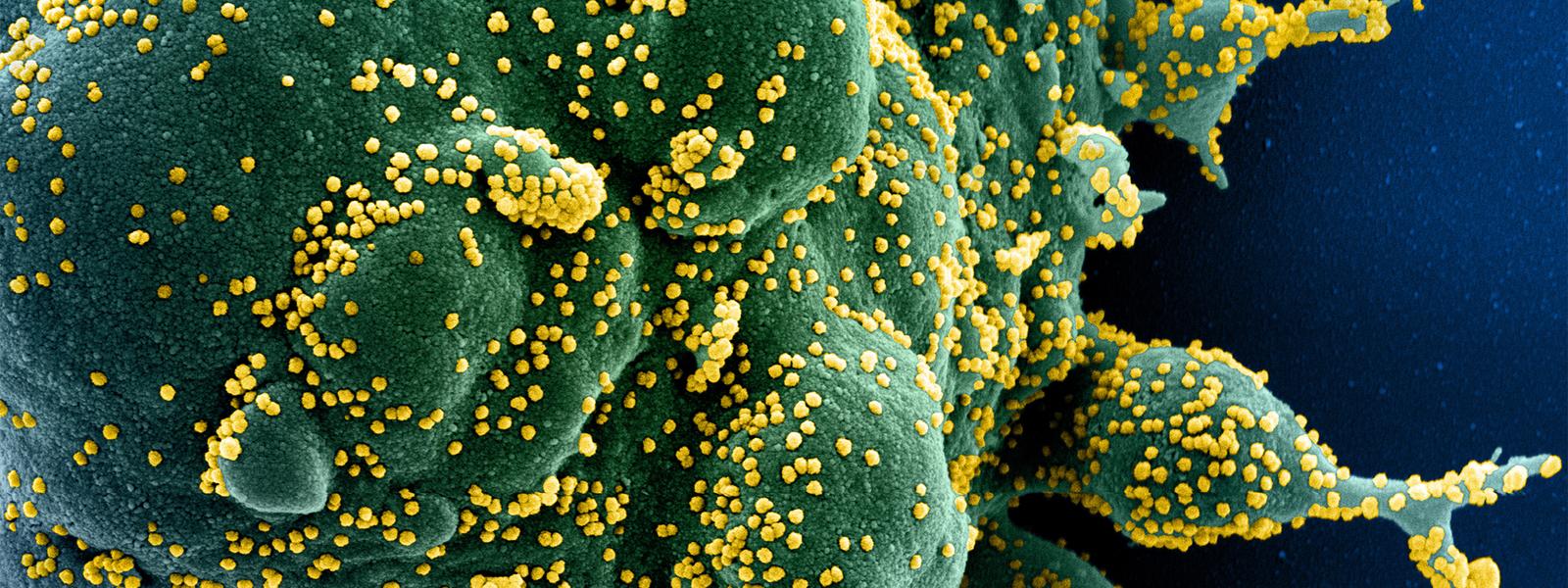
The success of today’s COVID-19 vaccines has given medical nanotechnology a long-awaited boost, with a renewed interest in its potential for treating disease among big pharma and small start-ups.
Over the years, nanotechnology has had its ups and downs in the public eye, cycling from peaks of excitement and fanfare–and a little too much hype–to falling out of favor because of individual product setbacks, said Steve Stern, Ph.D., co-director of the National Cancer Institute’s Nanotechnology Characterization Laboratory (NCL), located at the Frederick National Laboratory. Notable product failures have been targeted delivery systems, such as Avidimer’s folate targeted methotrexate dendrimer and Bind Therapeutics’ PSMA targeted docetaxel polymeric nanoparticle, which failed at preclinical and clinical stages. Many outside the field gave up on its promise.
Meanwhile, the science of building sophisticated medical technologies at the nanoscale has steadily evolved, tackling challenges that hindered its advancement. These include difficulties in product physicochemical characterization to determine product uniformity, which affects product performance, and the need for preclinical models and assays that can predict product clinical safety and efficacy. Researchers, including those at NCL, have spent many years on nanoparticle characterization, formulation scale-up, safety and other fundamental issues that have stood in the way of nanomedicine development and regulatory approval.
They have developed a diverse range of effective nanoparticle drug delivery systems, such as nanotechnology-based vaccines. So, when SARS-CoV-2 transmission became a pandemic, the stage had already been set for a rapid use of nanoparticle technology as a delivery system for the mRNA vaccines.
“Nanotechnology has a long history of use in drug delivery and medicine with many products approved for the clinical use,” said Marina Dobrovolskaia, Ph.D., NCL’s co-director. “Most of the (nanotechnology) products approved during the last decade are intended for cancer therapy; however, there are products for other indications as well.”
Established nanomedicine technology helped speed COVID vaccine
Pfizer and Moderna needed to get mRNA encoding the SARS-CoV-2 spike protein safely and effectively into the human body to fight the spread of coronavirus-induced disease. Using an established method of encapsulating therapeutic nucleic acid compounds in lipid nanoparticles, they rapidly produced vaccines for testing and regulatory approval.
And because these vaccines were deployed on such a large scale, their widespread success with relatively few side effects has attracted the attention of public and private funding sources, giving the entire nanomedicine field a new boost. Stern calls it a renaissance.
“It’s easier to get on the market with a proven technology that has a clear regulatory pathway,” Stern said. “Investors detest uncertainty, especially regulatory uncertainty. The coronavirus vaccines provided a new regulatory pathway. Now you see a lot of money going into venture capital investment and company start-ups.”
Dobrovolskaia sees it as an ongoing ebb and flow.
“Any new technology that is untried has an ‘S’ shape development curve,” she said. “First there is no activity, then exponential activity, then those who wanted too much too soon are discouraged, so activity slows down until something like COVID happens, then it gets a new life.”
Dobrovolskaia and Stern are encouraged by the burst of interest in nanotechnology but also know the ups and downs of the S curve. While engaging in the urgency of the moment, they are also dedicated to the methodical scientific studies that are the hallmark of enduring progress in a field.
Applied preclinical research is fundamental to optimizing nanomedicine safety and efficacy, resulting in delivery platforms with reduced toxicity and side effects and better biodistribution that will ultimately advance the therapeutic potential of the drug.
National push for enhanced nanomedicines means demand for formulation
Meanwhile, both large and small drug developers across the country are working to improve upon the early lipid nanoparticle vaccine carriers. The goals include increasing potency, minimizing side effects, and making these nanomedicines more effective in hitting their cellular targets.
“There have been a lot of new startups in past couple of years,” Stern said. “This is good and bad for us. We certainly appreciate the renewed interest in the field, but the concentration on lipid nanoparticle technology has the potential to starve other platforms of needed developmental resources. Also, we’re now facing staffing competition from these companies. We have to retain qualified staff, but the technology is hot and opportunities attractive.”
A big issue in nanomedicine is formulation. NCL is working with many outside laboratories to solve formulation problems so the drugs can be safely administered and reach their intended target tissues, releasing their therapeutic payload when and where they should, while also persisting long enough to have the desired effect. All this must happen using the lowest possible dose and with the fewest side effects.
Drug inventors often publish outstanding early data, based solely on in-vitro systems, and then they want to take their ideas directly into preclinical drug development. For that, they need effective drug formulations.
“Without decent formulation you can’t get into clinical trials,” Stern said.
More than lipid nanoparticles in technology toolbox
A lot of the current focus is on lipid nanoparticles, thanks to the coronavirus vaccines. But over the past 20 years, NCL has carefully characterized more than 450 unique nanomaterials with diverse potential medical applications, including carbon nanotubes, fullerenes, polymeric micelles and prodrugs, metallic/metalloid nanoparticles, lipid-based nanoparticles, nucleic acid and peptide-based nanoparticles, and dendrimers. NCL has helped get 17 of these nanomedicines into clinical use to benefit patients.
So, the true potential of medical nanotechnology reaches well beyond the currently celebrated lipid nanoparticle platform. And with the “S” curve in mind, NCL continues to advance nanoparticle characterization methodology and offer a range of scientific services including free comprehensive preclinical nanoparticle characterization services to the nanomedicine community. NCL also offers collaborative research opportunities, shared laboratory protocols, and professional development workshops and training.
“Whenever a new technology is introduced, the field [drug development] tends to focus on that particular idea, and the majority follows down that path,” wrote Kinam Park and colleagues in the December 2021 issue of the Journal of Controlled Release. “This is where diversity in research ideas becomes very narrow, and the field does not evolve.”
“Since lipid nanoparticles seem to be today’s research fashion, most research will focus on a similar subject,” the scientists wrote. “This may hinder trials of new ideas. Remember that no single tool is suitable for everything.”
Media Inquiries
Mary Ellen Hackett
Manager, Communications Office
301-401-8670
